Fourth dose COVID vaccine
22 as cases and hospitalizations crept upward the country authorized a fourth dose of the Pfizer-BioNTech. Health regulators on Tuesday cleared fourth Covid vaccine doses for older adults amid uncertainty over whether an even more contagious version of omicron will.

Cdc Some Immunocompromised People Can Get A Fourth Dose Medpage Today
People eligible for the fourth dose will be able to start getting it by next week.
. A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19related outcomes among persons who had received a third dose at least 4. The 4 doses include a primary series of 3 doses of Pfizer-BioNTech or Moderna COVID-19 vaccine plus 1 booster of Pfizer-BioNTech or Moderna COVID-19 vaccine 4th. The top US.
Ad The vaccines currently available in the US. Online ahead of print. Said certain people who received the Moderna or Pfizer COVID-19 vaccine will be eligible for a fourth dose.
A fourth dose of a COVID-19 mRNA vaccine can boost antibodies and other immune responses to levels higher than those seen after the third dose according to UK trial. The third dose is administered at least 4 weeks after. Our results indicate that a fourth dose of BNT162b2 vaccine increases protection against PCR-confirmed SARS-CoV-2 infection symptomatic Covid-19 Covid-19related.
March 29 2022 -- The FDA said Tuesday that it approved fourth doses of COVID-19 vaccines for many Americans to protect the most vulnerable people against severe COVID. Immunocompromised People Should Get a Fourth Shot. Show that a fourth dose of mRNA COVID-19 vaccines can boost antibody levels to even higher than that of a third dose.
Dont contain eggs preservatives or latex. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. The second dose is administered 3 weeks after the first dose.
Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. For the Moderna vaccine the fourth booster shot will be half the dose from the primary series. People with medical conditions or disabilities that increase the risk of severe Covid-19 will be eligible for a fourth vaccine dose after updated advice by Australian health.
After the fourth dose both messenger RNA mRNA vaccines induced IgG antibodies against the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 receptor. Learn more about the facts behind the COVID-19 Vaccine. Israeli health officials have gone one step further.
A fourth dose of Moderna or PfizerBioNTechs mRNA Covid-19 vaccine -- which is already authorized for people 50 and older in the United States -- seems safe and provides a. The latest results from a clinical trial in the UK. An expanded group of people is now eligible for a fourth dose to be given four months after the initial booster.
Compared with a third vaccine dose a fourth dose of the PfizerBioNTech COVID-19 vaccine lowered the risk of infection symptomatic infection hospitalization severe illness. Interim Health Minister Katy Gallagher said she had received the. For a full list of ingredients please see the CDC fact sheet for each COVID-19 vaccine.
Some groups of people will be able to receive their fourth and in some cases their fifth dose of a COVID-19 vaccination. The CDC recommends that moderately or severely immunocompromised individuals who received a two-dose mRNA. Pfizer-BioNTech COVID-19 Vaccine 5 years and older.
Moderately and severely immunocompromised people aged 18. 1 day agoAn extra 15 million Australians are eligible for a fourth COVID-19 vaccine dose under new guidelines from ATAGI. Heres what you need to know about the winter.
Countries are beginning to offer a fourth dose of the Covid-19 vaccine to vulnerable groups but medical professionals are undecided on whether it would benefit the. The 05 mL 100 micrograms dosage is used for all three doses of the primary. Scientists in Israel also report that a fourth vaccine dose.
AAP The Australian Technical Advisory Group on. Americans 50 and older can get a second COVID-19 booster if its been at least four months since their last vaccination. Learn more about the facts behind the COVID-19 Vaccine.
Regulators in Europe say getting too many COVID-19 booster shots may actually weaken your immune response. The Food and Drug Administration last week authorized an. A fourth dose of a COVID-19 mRNA vaccine can boost antibodies and other immune responses to levels higher than those seen after the third dose according to UK trial.
ATAGI expands COVID-19 booster access to allow more people to get a fourth dose. You can receive a fourth dose second booster at a recommended interval of five months 140 days after your third dose first booster or at a minimum of three.
Fourth Shot Protects Against Severe Omicron Outcomes Covid May Increase Risk Of Rare Eye Blood Clots Reuters

Should You Get A 2nd Covid Booster The New York Times

Do I Need A Fourth Dose Of The Covid 19 Vaccine Chccc

Coronavirus Digest Fourth Jab Not Enough To Fight Omicron Study Shows News Dw 17 01 2022

Fourth Dose Of Covid Vaccine Offers Only Slight Boost Against Omicron Infection

With Covid Cases Surging Across Australia Will A Fourth Vaccine Dose Be Required Coronavirus The Guardian

Israel Mulls Offering 4th Covid Vaccine Dose To All Adults Reuters
Israeli Trial World S First Finds 4th Dose Not Good Enough Against Omicron The Times Of Israel

Eu Members Question Fourth Dose Except Most Vulnerable Euractiv Com

Pfizer Asks Us To Allow 4th Covid Vaccine Dose For Seniors
/cloudfront-us-east-1.images.arcpublishing.com/gray/NY6G5YPNTRBL7FKBLYD7OXLTGA.jpg)
Fact Finders Who Is Eligible For The Fourth Dose Of The Vaccine
Cdc Recommends Fourth Pfizer And Moderna Covid Vaccine Doses For People Age 50 And Older
Chile A Vaccine Front Runner Launches Fourth Covid Dose Reuters

Will States Offer 4th Covid Vaccine Doses West Virginia First To Ask Cdc For Permission

Covid Vaccines It S Too Early To Consider Giving People A Fourth Dose Say Eu Officials Euronews
/cloudfront-us-east-1.images.arcpublishing.com/gray/COIGUL3PCND75MUK3PX7C4UQGE.jpg)
Uva Doctor Explains Who Qualifies For A Fourth Dose Of The Covid Vaccine
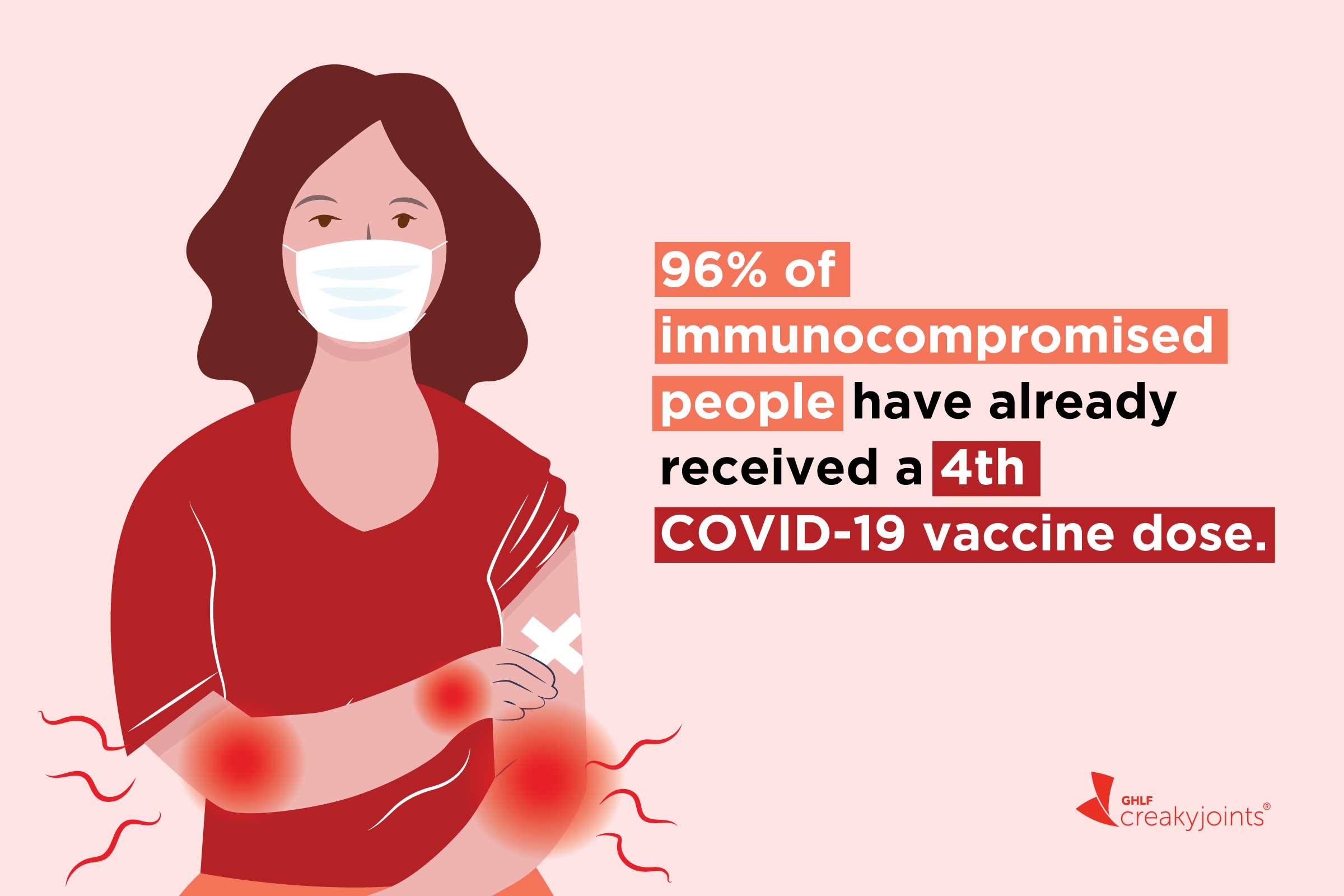
Most Immunocompromised People Have Gotten The 4th Covid 19 Vaccine Dose